The Harmful Algal Blooms (HABs) Misconception
Introduction: Why this fear exists
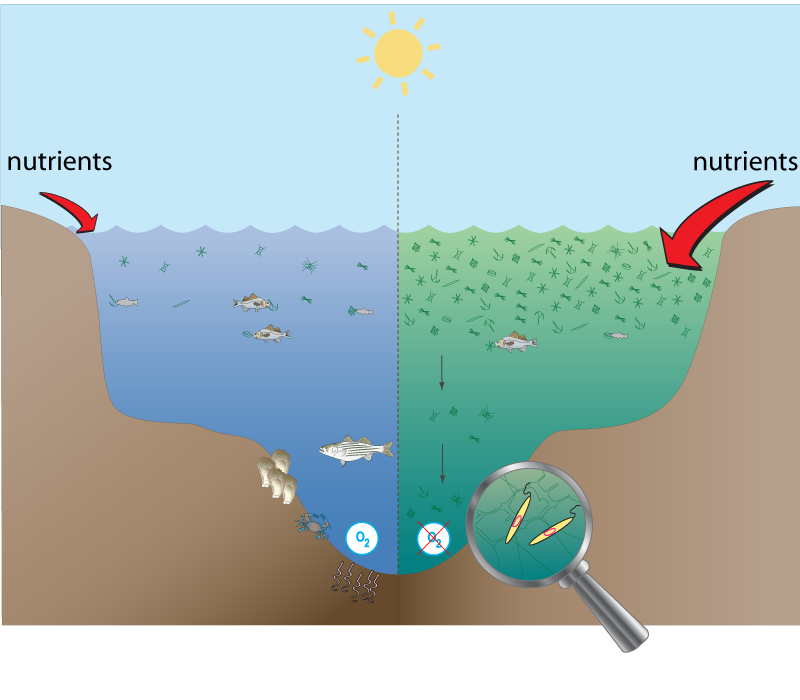
When people hear about adding nutrients to the ocean, they instinctively imagine harmful algal blooms (HABs) — toxic red tides, fish kills, and shellfish poisoning events that plague polluted coastal waters. It sounds intuitive: more nutrients mean more algae, more algae mean more problems. But this reasoning collapses once we distinguish between coastal eutrophication (runoff-fed, oxygen-starved systems) and open-ocean iron fertilization (trace-metal relief in vast, well-ventilated, iron-starved waters). The two systems are opposites in chemistry, ecology, and physics. In fact, every major scientific OIF experiment — from IronEx II to SOFeX, SEEDS, SERIES, and LOHAFEX — has shown the opposite of what HAB fears predict: healthy diatom growth, strong grazing by zooplankton, no toxins, and no fish or mammal deaths.

What toxic algae actually are
The phrase “toxic algae” describes a very small fraction of all phytoplankton. Most marine microalgae are harmless, photosynthetic base organisms that sustain life in the sea. Only a few species — mainly dinoflagellates like Alexandrium catenatum or certain diatoms in the genus Pseudo-nitzschia — occasionally produce molecules that can harm higher organisms. These toxins are secondary metabolites, not deliberate poisons. They evolved as part of predator–prey defense systems, not to “attack” humans or fish.
Saxitoxin, produced by Alexandrium, blocks sodium channels in nerve cells — a biochemical accident that affects humans who eat contaminated shellfish.
Domoic acid, sometimes produced by Pseudo-nitzschia, mimics neurotransmitters and can cause a condition called amnesic shellfish poisoning, which affects memory.
But these molecules do not occur everywhere or all the time. Even the same species may or may not produce them depending on environmental stress, nutrient ratios, and temperature. They are rare chemical quirks, not a general property of phytoplankton.

Where harmful blooms really occur
All documented large-scale toxic algal events come from coastal, nutrient-overloaded, and stratified regions — not the open ocean. Typical hot spots include:
- The Gulf of Mexico, where river runoff full of nitrogen and phosphorus fuels dense, decaying blooms.
- Scandinavian fjords, where calm, layered water allows Chrysochromulina polylepis to form dense mats that suffocate fish.
- California and Portugal coasts, where Pseudo-nitzschia blooms occasionally produce domoic acid affecting sea lions or shellfish consumers.
These blooms need macronutrient oversupply, low mixing, and retention — the very conditions absent in OIF regions like the Southern Ocean or equatorial Pacific. There, the surface waters are iron-limited, cold, and mixed by storms and westerly winds. Adding a few milligrams of iron per square kilometer merely allows the system to use the existing nitrate and phosphate that were already unused — it doesn’t overload anything.
Why OIF cannot trigger harmful algal blooms
OIF adds a missing micronutrient, not excess macronutrients.
The Southern Ocean and equatorial Pacific are rich in nitrate and phosphate but poor in iron. Iron is added only in trace amounts, typically raising dissolved iron levels to 0.000056–0.000112 mg per litre —
about 2,500–5,000 times below the World Health Organization’s drinking-water guideline of 0.3 mg per litre, and
thousands of times below any ecological stress threshold (~0.21 mg per litre).
These concentrations are delivered as a very diluted liquid, not a dump of particles. The iron is complexed and dispersed within hours.
The physical environment prevents bloom stagnation.
In the open ocean, wind, waves, and circulation continually mix surface water. Unlike enclosed bays, there’s no stagnant layer for algae to accumulate or decay in. Even the largest OIF patches (50–300 km²) disperse naturally within weeks.
The species composition is different.
In every major OIF experiment, the bloom was dominated by non-toxic diatoms such as Chaetoceros, Thalassiosira, and Fragilariopsis. These are the same silica-shelled plankton that fuel krill, copepods, and fish larvae — not toxin producers.
In experiments like EIFEX and LOHAFEX, Pseudo-nitzschia was present but produced no domoic acid, verified by laboratory analyses at the Max Planck Institute. The same genus in temperate coastal waters behaves differently because of species variation and environmental triggers.
There is no oxygen depletion or “dead zones.”
In eutrophic coasts, massive decaying blooms consume oxygen. In OIF regions, the surface is highly ventilated and the organic matter sinks quickly, preventing oxygen loss. Every OIF expedition measured oxygen directly, and none recorded any decline.
No evidence from field data.
Across decades of global iron fertilization research, there has never been a single verified case of fish, mammal, or bird mortality linked to OIF. Instead, scientists consistently found enhanced grazing, carbon export, and ecosystem stability.
Revisiting the domoic acid scare
The fear of domoic acid poisoning derailed several proposed OIF experiments after 2012, but the scientific basis is weak. The only serious human event occurred nearly 40 years ago in the Gulf of St. Lawrence, Canada — far from any iron-limited region. Three elderly patients in a nursing home died after eating contaminated mussels; around 100 others recovered. That was a coastal bloom in a temperate estuary, not an open-ocean process.
Since then, no comparable incident has been recorded anywhere else in the world. Even off California, where Pseudo-nitzschia species are common, domoic acid events affect only small numbers of sea lions each year — while the population overall continues to grow steadily.
In the Southern Ocean, multiple campaigns — including those led from the research vessel Polarstern — tested for domoic acid and found none at all. The absence of domoic acid in hundreds of samples rarely makes the news, but it is the dominant result. Only two isolated detections have ever been published from the entire Southern Ocean, while many other studies reporting “zero” remain unpublished because “negative” results are undervalued in science.
The conclusion from decades of fieldwork is simple: there is no measurable domoic acid problem in the regions targeted for OIF.
Why the misconception survives
The persistence of the HABs misconception around OIF comes from three overlapping biases:
Media bias toward fear.
News about “killer algae” spreads faster than “nothing happened.” Images of stranded sea lions and red tides are emotionally powerful, even if they are unrelated to open-ocean conditions.
Scientific publication bias.
Researchers rarely publish “no toxin found” data. A single positive detection somewhere can generate dozens of citations, while hundreds of negative results remain invisible.
Funding and institutional incentives.
Marine toxin research attracts legitimate funding for seafood safety. The field naturally amplifies risk awareness. As a result, reports that emphasize danger receive more visibility than those confirming stability.
This creates a distorted public perception — one in which HAB risk appears universal, when in fact it is extremely localized and conditional.
Ecological perspective: toxins as part of evolution, not a disaster
It is also worth remembering that the natural world is full of chemical defenses. Many common land plants are toxic if eaten — foxglove, oleander, rhododendron — yet they are part of healthy ecosystems. The ocean is no different: phytoplankton evolve molecules to deter grazers, just as plants do with slugs or insects. Most of these compounds have no relevance to humans or whales, and their sporadic presence does not signal ecosystem collapse.
Scientific consensus from past OIF experiments
Here’s what decades of OIF fieldwork have actually shown:
- No toxic algae outbreaks in any fertilized patch.
- No increase in harmful or nuisance species.
- No evidence of domoic acid, saxitoxin, or other toxins.
- Strong diatom growth, healthy grazing by krill and copepods, and enhanced carbon export.
- No oxygen depletion, no pH stress, and no fish or whale deaths.
These outcomes were replicated across the equatorial Pacific, subarctic Pacific, and Southern Ocean by teams from the U.S., Germany, Japan, Canada, and India. The ecological responses were consistent, predictable, and entirely non-toxic.
The real takeaway: restoration, not risk
Ocean Iron Fertilization is not “feeding poison blooms.” It is restoring a broken nutrient cycle. By supplying trace iron in a safe, diluted, and transient way, it revives the diatom–krill–whale loop that once kept the Southern Ocean teeming with life. The iron quantities are thousands of times below any toxic limit, delivered as a very diluted liquid, and quickly complexed and dispersed.
The open ocean is vast, dynamic, and self-buffering. It cannot experience coastal-style eutrophication. OIF works within those natural constraints — stimulating life, not suffocating it.
Conclusion
The belief that Ocean Iron Fertilization could trigger harmful algal blooms is scientifically unfounded. Toxic blooms are a coastal phenomenon driven by excess nitrogen, phosphorus, and stagnant water — the exact opposite of OIF’s setting and method.
In over 30 years of experiments, no harmful bloom, no toxin spike, and no ecosystem damage have ever been observed following iron addition. Instead, OIF consistently supports healthy diatom growth, improved food-web productivity, and carbon export. The HAB fear belongs to river mouths and estuaries — not the vast, iron-limited expanses of the Southern Ocean where OIF operates.
In short, OIF does not create toxic blooms; it restores life where iron scarcity has silenced it.
← Back to all misconceptions