The Nutrient Robbing Misconception
Why the “robbing” story sounds plausible—but isn’t
Critics often argue that fertilizing iron-limited waters like the Southern Ocean will steal macronutrients (nitrate, phosphate, silicic acid) that would otherwise upwell elsewhere to support fisheries. The intuition is: “If you use nutrients in the south, there’s less left for the east-Pacific upwelling or Benguela/Canary systems.” That framing treats the ocean as a static, zero-sum reservoir. In reality, the ocean is a circulating, regenerating machine: organic particles sink, decompose at depth, and recharge deep waters with nutrients that re-enter the sunlit layer via upwelling and mixing—again and again. OIF doesn’t purge nutrients from the ocean; it re-routes when and where they are used, often reducing excesses that currently drive oxygen loss and greenhouse-gas production.
The global nutrient conveyor—what really moves the “food”
The deep ocean is the bank; the surface is the spending layer
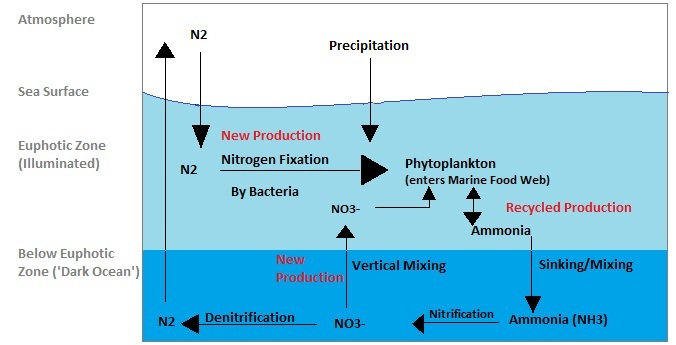
Deep waters store the bulk of macronutrients. They are “refilled” by sinking particles (the biological carbon pump) that remineralize at depth, returning nitrate (NO₃⁻), phosphate (PO₄³⁻), and dissolved silica (Si(OH)₄) back to the water column.
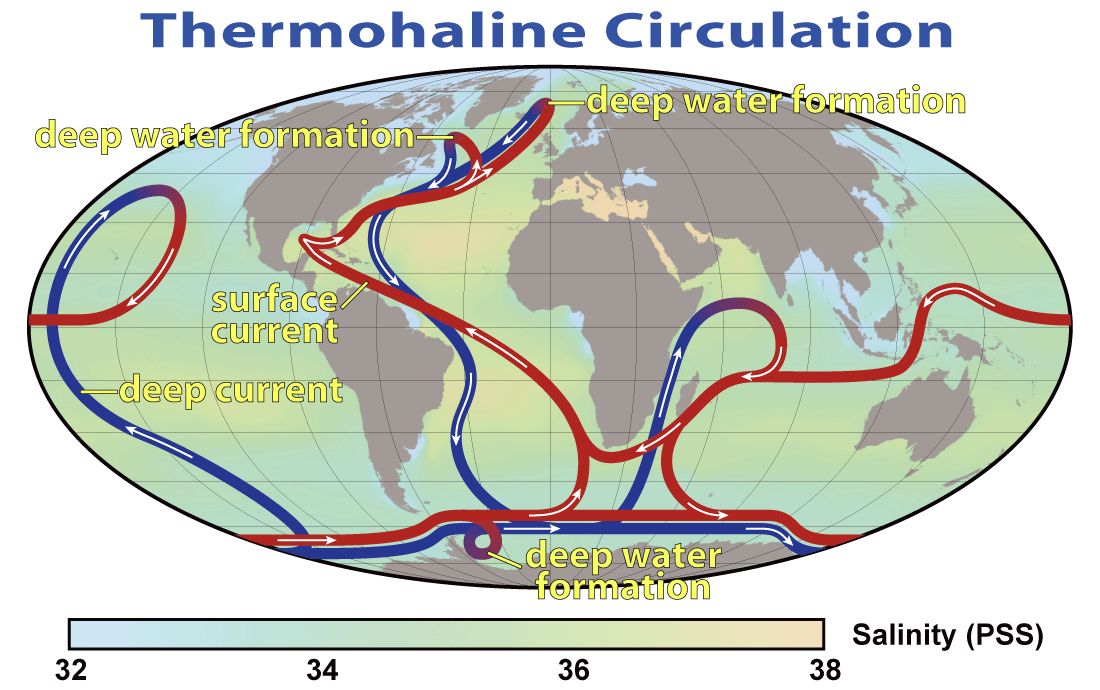
Upwelling (equatorial divergence, high-latitude wind-driven upwelling, coastal eastern boundary systems) and mixing are the ATM withdrawals that bring nutrients back to light.
The two key sources of deep/ventilated waters
North Atlantic Deep Water (NADW): Forms in the North Atlantic and sinks southward, carrying oxygen and relatively low “preformed” nutrients.
Antarctic system:
Antarctic Bottom Water (AABW): Very dense, formed mainly in the Weddell and Ross sectors; it ventilates abyssal oceans.
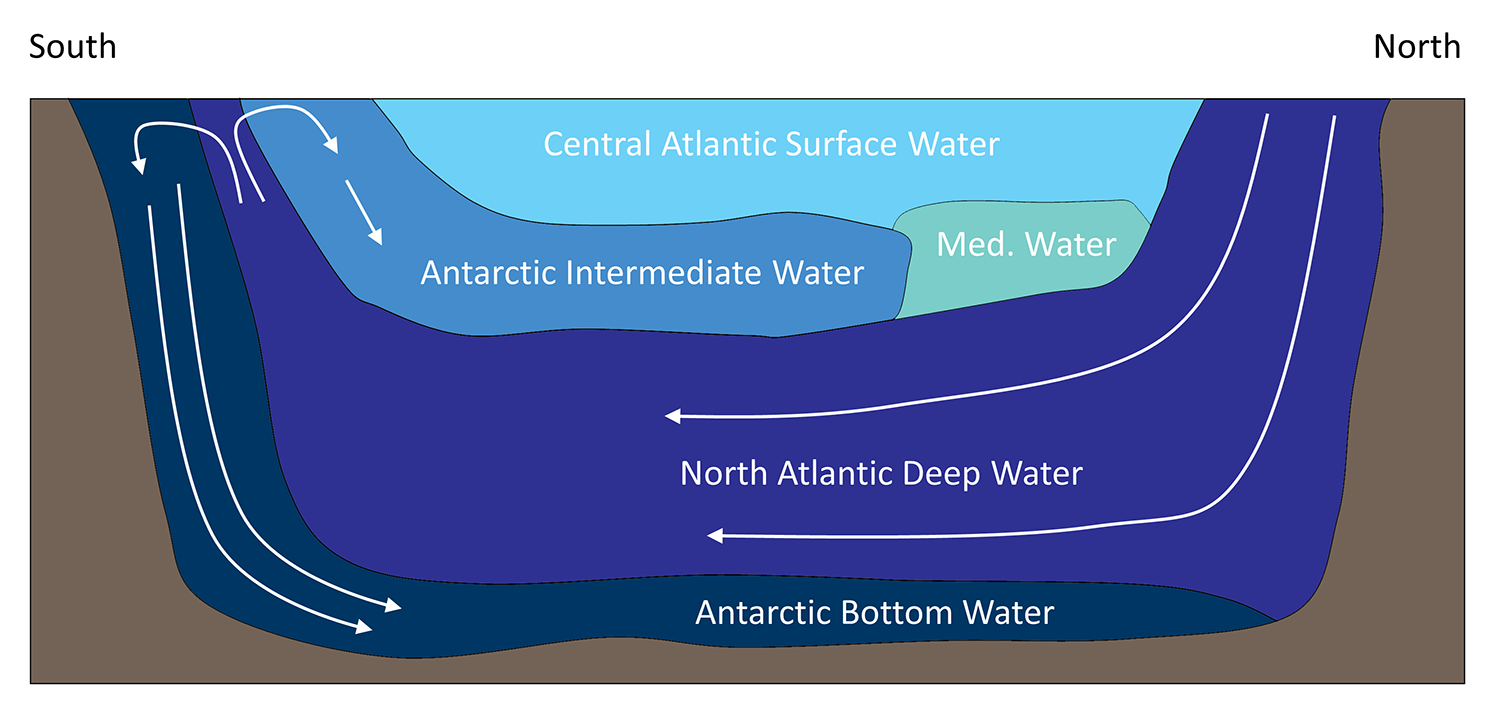
Antarctic Intermediate Water (AAIW): Forms on the northern rim of the Antarctic Circumpolar Current (ACC), subducts beneath warmer subtropical waters, and spreads northward into all basins.
The specific “15 → 30 → 40–45” nitrate story (keeping your numbers intact)
Going down (today): About 15 mmol m⁻³ nitrate subducts in AAIW (a measure of preformed nitrate—nutrients that escaped surface biological use).
Coming up (later): Upwelled waters along continental margins and the equator commonly carry ~30 mmol m⁻³ nitrate.
Oldest waters (North Pacific): Reach 40–45 mmol m⁻³—because they’ve had the longest time at depth to accumulate regenerated nutrients from sinking particles.
Implication: As water ages at depth, it accumulates nutrients. Even if you hypothetically drove “going-down” nitrate toward zero (you cannot in practice), deep waters would still recharge during their multi-decadal/centennial journeys before they upwell again. Nutrients are recycled, not “lost.”
Southern Ocean structure—where nutrients are “stuck,” where they aren’t
HNLC logic (High-Nutrient, Low-Chlorophyll)
The Subantarctic and Antarctic zones of the Southern Ocean are classic HNLC regions: macronutrients abound but iron is scarce, so phytoplankton don’t fully use nitrate and phosphate. This creates large pools of “preformed” nutrients that subduct into AAIW and travel north.
The “nutrient trap” concept
Because export production (sinking particles) in the Southern Ocean is substantial, a lot of organic matter remineralizes at depth and re-enters the surface south of the subtropical fronts. This can keep a latitudinal band in a state where nutrients are repeatedly cycled without being efficiently converted into long-lived biomass and carbon export—a trap in the sense that macronutrients persist unused in the surface/subsurface here compared with iron-replete regions.
Where Southern Ocean nutrients flow into non-iron-limited regions
AAIW formed around the ACC’s northern rim is the source water for:
Eastern boundary upwelling systems (EBUS): Benguela (Namibia/SW Africa) and Canary (NW Africa), plus California and Peru/Chile.
Equatorial divergence (Pacific/Atlantic): Continuous upwelling with high primary productivity.
These downstream regions are not iron-limited the way the Subantarctic/Antarctic zones are. They often receive more macronutrients than food webs can safely process—with oxygen minimum zones (OMZs) and eutrophication problems as a result.
When “too many nutrients” harms ecosystems (your Namibia example, expanded)
In places like the Namibian shelf (Benguela), excess nutrients drive intense blooms; as biomass sinks and decomposes in weakly ventilated waters, oxygen plummets and anoxia develops. Consequences include:
- Mass invertebrate escapes/die-offs: The “lobsters crawling out of the ocean” event you referenced is emblematic of seafloor hypoxia/anoxia.
- Greenhouse gases: Anoxic/suboxic waters promote methanogenesis (CH₄) and denitrification/anammox, producing nitrous oxide (N₂O)—both far more potent than CO₂ per molecule.
- Food web stress: Fish and invertebrates are compressed into thin oxygenated layers; recruitment and habitat quality suffer.
In these contexts, slightly reducing the nutrient load that eventually feeds such upwelling systems can improve ecosystem condition—less anoxia, fewer extreme events, lower CH₄/N₂O—without meaningfully reducing sustainable fish production (because the current supply already exceeds what can be cleanly processed).
What OIF actually does in iron-limited waters
Triggering diatoms where they’re starved of iron
In HNLC regions, iron is the missing micronutrient. Adding small, controlled doses (e.g., iron sulfate) can unlock macronutrient use, especially by silicifying diatoms (provided silicic acid is available).
Diatoms package carbon efficiently into faster-sinking aggregates and fecal pellets, enhancing export below the winter mixed layer—reducing the chance of rapid re-ventilation.
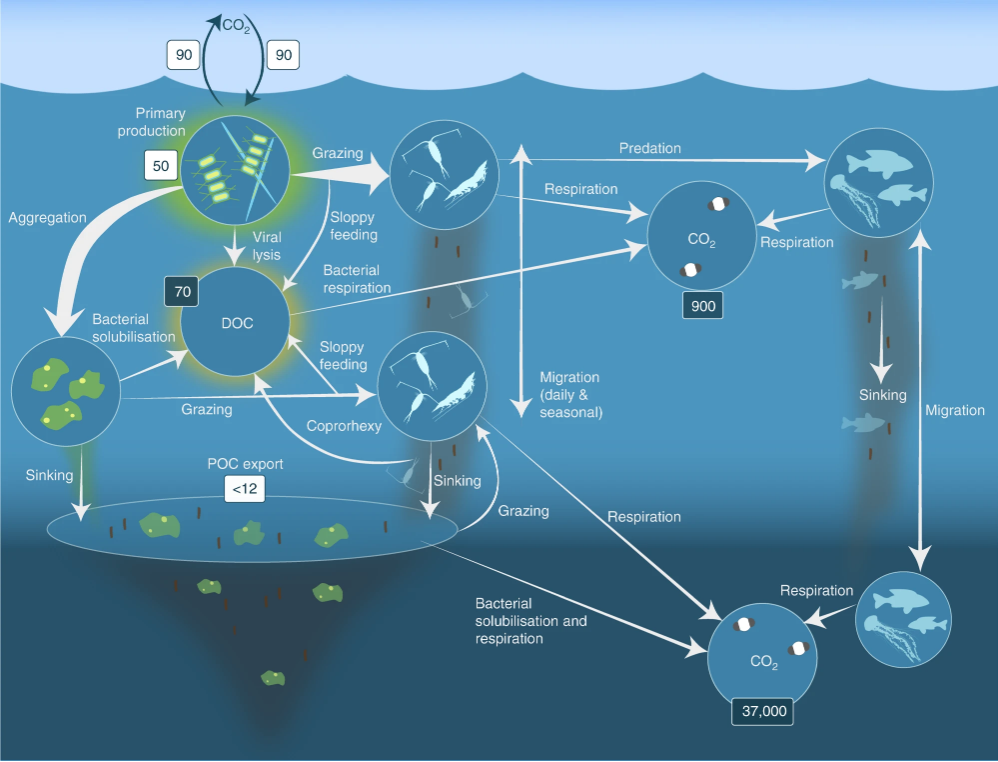
The Redfield-plus-iron stoichiometry reality
Canonical Redfield (C:N:P ≈ 106:16:1) describes bulk needs, but Fe:C demand is tiny (μmol:mol range) and ecotype-dependent. In the Southern Ocean, Fe:N and Fe:P ratios are limiting; small Fe inputs can mobilize large N and P draws.
Silica matters: Diatoms also need Si:N ≈ 1:1 (very roughly) to build frustules; Si availability and light (day length, mixed-layer depth) co-limit bloom magnitude and timing.
Why you “can’t take all the nutrients”
Even with iron addition, not all nitrate/phosphate is consumed because:
- Light limitation & deep mixed layers in austral winter/spring cap growth.
- Si limitation can arrest diatom dominance before nitrate is exhausted.
- Grazing & viral lysis recycle nutrients within the surface.
- Physical export ceilings: Stratification, storms, and fronts limit bloom residence times.
Hence the hypothetical “zero going down” is not achievable at scale—and even if it were, the regeneration at depth (particles raining down) would recharge nutrient inventories before waters re-upwell (your “15 going down, ~30 coming up” logic).
Addressing the big fear: “Are we depriving fisheries?”
Upwelling regions already receive excess macronutrients
Equatorial and EBUS systems often have more NO₃⁻/PO₄³⁻ than cleanly utilizable, creating OMZs and GHG hotspots (CH₄, N₂O). Slightly downsizing the nutrient pulse that arrives decades later can reduce extremes without starving food webs.
Only a small fraction of nutrients ultimately becomes harvestable fish
Most nutrients cycle microbially or are respired; only a minor share supports higher-trophic biomass. The idea that “every nitrate molecule must feed a fish” is romantic but wrong. In oversupplied regions, marginal reductions in far-downstream nitrate help rather than hurt.
Win–wins: lower CH₄/N₂O + fewer low-oxygen crises
OIF that taps HNLC surplus near fronts/mesoscale features can convert unused surface macronutrients into exported carbon, lowering the burden of downstream eutrophication and reducing the formation of anoxic layers—clear ecosystem and climate benefits.
Where in the Southern Ocean OIF makes the most sense
“Stuck” nutrients to target
Subantarctic Zone (SAZ) north of the Polar Front: Chronic Fe limitation, frequent unused NO₃⁻/PO₄³⁻/Si in the surface layer, strong AAIW subduction—ideal for converting preformed nutrients into export.
Polar Front eddy fields & meanders: Natural retention enhances bloom development; diatom-friendly silica is often adequate early in the season.
Avoiding areas where nutrients already flow to non-Fe-limited sinks
Sectors where rapid isopycnal pathways couple directly and quickly to heavily utilized downstream regimes can be de-prioritized if the aim is to target surplus nutrients that would otherwise subduct unused.
Seasonality, light, and mixed-layer physics
Late winter/early spring windows (shoaling MLD, increasing light) allow efficient Fe use and export before strong stratification or silica drawdown stalls diatoms.
Site choice weighs silicic acid fields, front locations, eddies, and storm climatology to favor exportable diatom blooms over short-lived recycled spikes.
Quantitative ceilings on annual diatom build-up (what limits scale)
Even with steady upwelling, annual bloom potential in the Southern Ocean is capped by:
- Iron delivery (added + natural dust/ice melt + shelf inputs) and ligand chemistry (organic ligands keep Fe soluble; scavenging removes it).
- Silica inventories and Si drawdown rates (diatoms can deplete Si before NO₃⁻ is gone, flipping communities away from fast-sinking types).
- Light & mixing (deep winter MLDs dilute cells/Fe; storms re-mix and can terminate blooms).
- Top-down control (grazing, viral lysis) that redirects production into rapid remineralization rather than export.
- Physical export pathways (eddies/fronts that retain blooms long enough for aggregates to cross the “memory line”—below the winter mixed layer).
Bottom line: Nature itself enforces quantitative limits; OIF tunes where and when nutrients are used, not whether the entire Southern Ocean is stripped.
Climate-scale co-benefits that the “robbing” frame ignores
Lower CH₄/N₂O formation: By lowering excess organic matter delivery to already suboxic shelves/OMZs downstream, OIF can shave peaks of microbial processes that emit CH₄ and N₂O, both with high radiative forcing.
Exported carbon stays down longer: Sinking diatom aggregates that pass beneath the winter MLD delay contact with the atmosphere from years to centuries, depending on depth and circulation.
Oxygen relief: Less excess production in OMZ-adjacent waters = healthier oxygen budgets, fewer mortality events, improved habitat quality for fish/invertebrates.
Why the Namibia “lobsters on the beach” story matters
Your example illustrates the central point: more nutrients ≠ better fisheries once oxygen is pushed too low. In the Benguela, runaway productivity + stratified shelf waters → anoxia, sulfidic events, CH₄/N₂O pulses, and benthic/faunal stress. If the far-upstream nutrient pipeline is moderated slightly (because Southern Ocean HNLC pools are used in situ), the net outcome can be fewer extreme events and more stable production—not starvation.
Safeguards and design principles for responsible OIF
To ensure we’re converting surplus (unused) nutrients rather than competing with downstream productive systems, programs should:
- Target HNLC preformed-nutrient pools (SAZ/Polar Front) with clear Fe limitation.
- Time releases to coincide with light windows, shoaling MLD, and sufficient Si for diatoms.
- Use conservative dosing (tiny Fe:C leverage) with spatial containment (fronts/eddies) for retention.
- Instrument deeply (CTD/Chl-a/FRRf/²³⁴Th, sediment traps, zooplankton tows, O₂, CH₄, N₂O) to verify export, oxygen, and GHG outcomes.
- Track isopycnal fates (Lagrangian floats, dye/argo BGC) to confirm downstream effect sizes are neutral-to-beneficial.
- Stop rules: If oxygen stress or unintended signals arise, cease and reassess.
Reframing the narrative—optimization, not extraction
Keeping your original logic intact:
Today, about 15 mmol m⁻³ nitrate goes down unused in AAIW; upwelling later commonly shows ~30 mmol m⁻³ because regeneration adds nutrients at depth; the oldest waters hold 40–45 mmol m⁻³.
Therefore, using HNLC nutrients now (with Fe) does not deprive the future; it changes where/when nutrients are drawn down and can reduce harmful anoxia and CH₄/N₂O.
The idea that “every nutrient must remain in the surface cycle forever” ignores that excess nutrients already cause damage; moving a portion into exported biomass in iron-starved regions can be ecologically and climatically restorative.
What this means in practice
For climate: OIF in the right Southern Ocean bands reduces potent GHGs from low-oxygen zones and boosts carbon export.
For ecosystems: It can stabilize vulnerable upwelling margins by dampening extremes, improving oxygenation and habitat quality.
For fisheries: It doesn’t starve downstream systems because the present nutrient pipeline is over-generous relative to safe oxygen budgets; modest moderation is a benefit, not a theft.
For ethics/governance: With conservative dosing, hard monitoring, and clear stop rules, OIF is not robbing nutrients; it’s optimizing a misallocated surplus.
Take-home
The “nutrient robbing” critique assumes a fixed pot that only shrinks. The ocean’s nutrient economy is dynamic, recharging, and path-dependent. In the Southern Ocean, vast iron-limited pools allow macronutrients to subduct unused—your 15→30→45 mmol nitrate story captures precisely how regeneration refills that bank. Carefully sited OIF uses what is currently wasted, reduces downstream oxygen stress and GHGs, and does not deplete the ocean’s overall productive capacity. The question is not “are we stealing nutrients,” but “are we smartly converting surplus into durable climate and ecosystem gains?” With good design, the answer is yes.
← Back to all misconceptions