The Oxygen Depletion Misconception
Introduction
OIF is often framed by critics as a pathway to “dead zones”: add nutrients, spark a bloom, let it rot, and oxygen gets consumed until marine life suffocates. That chain of events is real in river-fed, semi-enclosed seas, but it is the wrong mental model for open-ocean, wind-mixed upwelling/HNLC regions where responsible OIF is considered. To separate fear from physics, we need to understand what an oxygen minimum zone is, where it forms, and why the circulation and ecology of these regions make chronic deoxygenation from OIF unlikely.

What an Oxygen Minimum Zone (OMZ) Actually Is
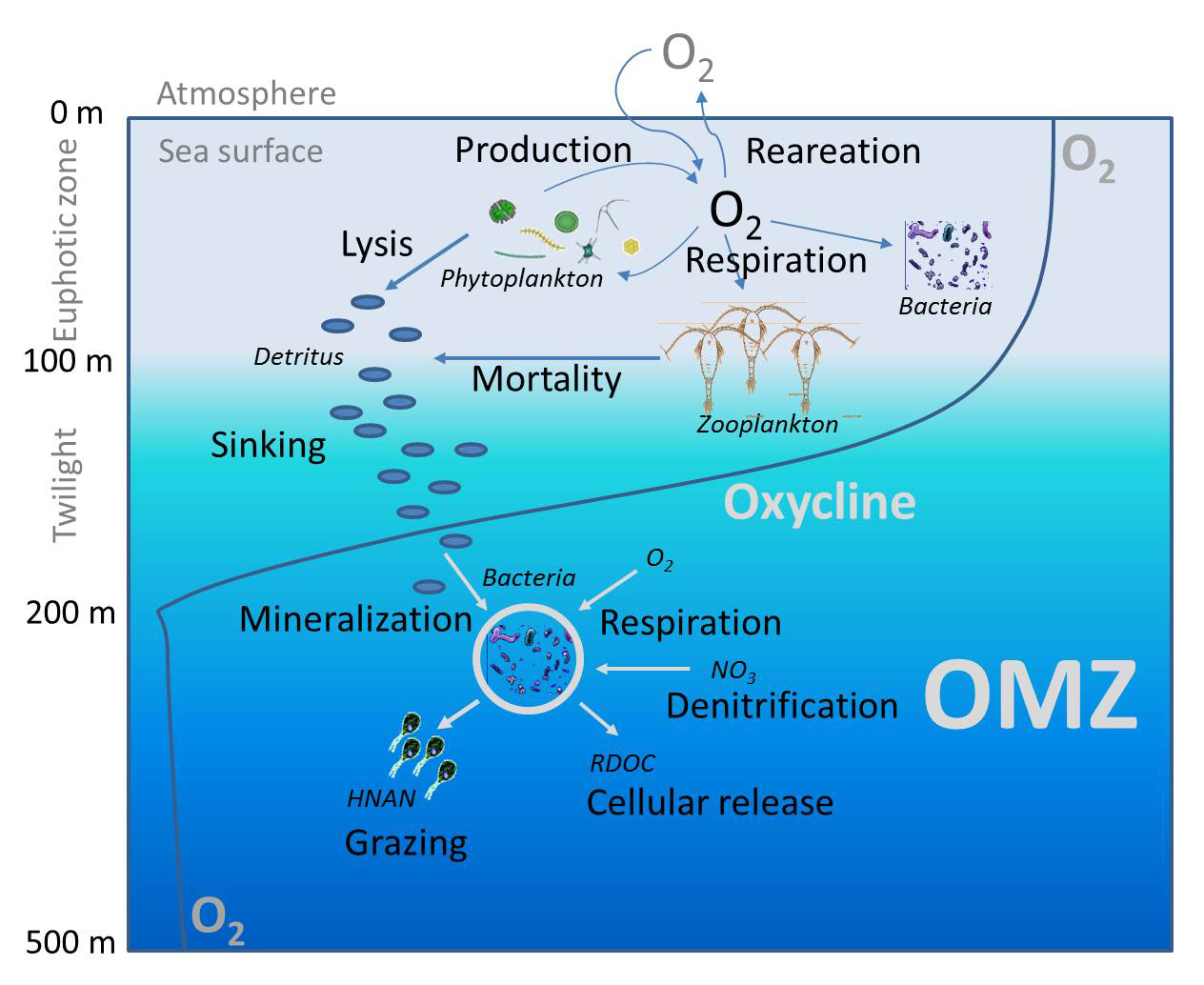
An OMZ is a layer beneath the well-mixed surface where sinking organic matter is respired faster than oxygen can be replenished. The surface layer is continually re-oxygenated by the atmosphere and by photosynthesis; the layer below is not. When respiration outpaces ventilation, oxygen dips. Under sufficiently low oxygen, microbes can generate methane (CH₄) and nitrous oxide (N₂O). These processes are real—but they are context-dependent. They thrive in waters that are stratified, poorly ventilated, and residence-time limited.
Why Open-Ocean Upwelling/HNLC Regions Don’t Fit Coastal “Dead-Zone” Analogies
Open-ocean upwelling/HNLC regions are vast, wind-mixed domains where waves, winds, and frontal mixing keep the mixed layer ventilated. Even when blooms occur, the energy in these systems continually refreshes oxygen in the upper ocean. That is fundamentally different from enclosed or semi-enclosed basins—the Baltic, the Black Sea, the northern Arabian Sea, the Bay of Bengal margins, or river-mouth shelves like the northern Gulf of Mexico—where stratification, restricted exchange, and long subsurface residence times allow oxygen debt to accumulate and persist.
Coastal Eutrophication vs. OIF: Different Inputs, Different Outcomes
Coastal dead zones are driven by macronutrient over-enrichment (nitrogen and phosphorus) from rivers, wastewater, and fertilizers. Blooms collapse, biomass decays in place, and bottom waters go hypoxic because stratification isolates them from the atmosphere. OIF does not add nitrogen or phosphorus; it adds trace iron in regions already replete with macronutrients but unable to use them because of iron scarcity (HNLC conditions). In these settings, iron additions typically favor diatoms—silica-shelled phytoplankton that form heavy aggregates and sink—which limits the accumulation of decaying biomass at the surface where oxygen exchange occurs.
“Won’t the Bloom Just Rot and Strip Oxygen?”
Two features of these open-ocean regions push against the coastal script. First, ventilation: frequent storms and wave breaking mix oxygen downwards, preventing sustained near-surface O₂ deficits. Second, export: diatom-rich blooms, once they peak, sink rapidly, carrying organic matter below the winter mixed layer (“the memory line”) where it no longer immediately competes with surface waters for oxygen. Some respiration occurs along that descent, but not in a way that creates a stagnant, bottom-trapped oxygen sink as in stratified estuaries and shelves. In short, export re-routes where respiration happens and decouples it from the ventilated surface.
CH₄ and N₂O: Real Pathways, Wrong Geography
Yes—low-oxygen waters can favor methanogenesis and denitrification/anammox that emit CH₄ and N₂O. But those pathways intensify where water is poorly ventilated and organic matter lingers. Properly sited OIF in open-ocean HNLC domains chooses ventilated, high-exchange environments, monitors O₂, CH₄, and N₂O directly, and employs stop rules if signals turn adverse. There is also a downstream benefit often missed in the debate: by using some of the surplus macronutrients in situ (where they would otherwise subduct unused), OIF can slightly reduce the nutrient load eventually feeding coastal upwelling systems that already suffer from oxygen stress, thereby dampening extreme anoxia and associated CH₄/N₂O production there.

Why Baltic, Black Sea, and Gulf of Mexico Stories Don’t Transfer
In the Baltic, deep waters are isolated; when they turn anoxic, phosphate and iron are remobilized from sediments, selecting for surface scums of nitrogen-fixing cyanobacteria under calm conditions. That is a residence-time and ventilation problem, not a “bloom first, oxygen second” chain caused by iron. In the Gulf of Mexico, a fresh, stratified Mississippi plume caps the shelf; nutrients fuel blooms above, while stagnant bottom waters lose O₂ below the cap. Again, it’s hydrography plus macronutrient loading, not trace-iron stimulation in an open, storm-mixed sea.
Community Composition, Grazing, and Why Diatoms Matter
Diatoms dominate when silicic acid is available and iron limitation is relieved. They are poorly preferred by many copepods (tooth wear is real), which helps diatoms escape grazing, form aggregates, and sink—a built-in export mechanism that reduces the chance of surface decay driving local hypoxia. Krill exploit this niche differently: they can seek diatom patches and crush frustules in their gizzard, routing some production to the food web without causing a surface-layer oxygen crisis. In either case, the accumulation of decaying material right where oxygen exchange occurs is limited.
Mixing by Life: The Overlooked Stabilizer
Large animals mix water. Historical swarms of menhaden, herring, and whales imparted turbulence that enhanced small-scale ventilation in coastal and shelf seas. Their depletion likely reduced biogenic mixing. In open-ocean HNLC regions, physical mixing (winds, fronts, eddies, storms) dominates, but recognizing biogenic mixing underscores a broader point: oxygen dynamics are shaped by movement and exchange, not simply by bloom magnitude.
Cyanobacteria and Causality: Which Comes First?
In systems like the Baltic, buoyant cyanobacteria often follow seasonal oxygen depletion; they exploit the iron- and phosphate-rich conditions that anoxia unlocks from sediments. They are symptoms of the physical and chemical state, not its root cause. Projecting that sequence onto open-ocean upwelling/HNLC regions reverses causality: there, the physical template prevents the stratified, long-residence conditions that let such scums dominate.
Design Choices That Keep Oxygen Safe
Responsible OIF avoids rapid-subduction corridors (where air–sea CO₂ equilibration would be short-circuited) and favors retentive yet ventilated mesoscale features (fronts, eddies) in iron-limited, silica-sufficient bands. It uses tiny, phased iron doses, intensive before–during–after measurements (O₂ profiles, community microscopy plus molecular assays, CH₄/N₂O, export tracers), and predeclared stop rules. The objective is not “bigger blooms at all costs,” but timed, diatom-leaning production that crosses the memory line without building a coastal-style oxygen debt.
Conclusion
Oxygen-depletion fears around OIF come from misapplying coastal eutrophication logic to open-ocean upwelling/HNLC regions, where the physics, chemistry, and ecology are different. In iron-limited, storm-ventilated waters, modest iron additions unlock existing macronutrients, favor diatom export, and do not create the stagnant, bottom-trapped oxygen crises familiar from river-dominated shelves and enclosed seas. With careful siting, conservative dosing, and live monitoring, OIF shifts production pathways away from surface decay and toward export, while potentially easing oxygen stress in downstream upwelling systems. The ocean is not a still tank; oxygen risk is governed by ventilation, residence time, and export—and on those fundamentals, these open-ocean regions do not fit the dead-zone script.
← Back to all misconceptions